TECHNOLOGY
Making Life-Changing Therapies More
Accessible Across A Wider Range of Patients
Lupagen is the only medical device and services company dedicated to bedside cell and gene therapy
Our mission is to expand access to innovative therapies by lowering cost and simplifying delivery
Lupagen can help biopharma companies that are exploring alternative delivery solutions to address current development and market challenges
HOW IT WORKS
Lupagen’s Bedside Delivery System
Lupagen Xynvivo™ System collects the patient’s peripheral blood cells using standard leukapheresis process.
Binding to transgene viral vector or nanoparticle product occurs within the Xynvivo™ System and unbound particles are removed prior to reinfusion to the patient.
This entire process is done in a patient-connected closed loop procedure at the bedside in a few hours.
PLATFORM BENEFITS
Extracorporeal Bedside Delivery Platform
with potential to improve Ex Vivo Cell
Therapy and In Vivo Gene Therapy
SERVICES
Lupagen is the only company dedicated to providing Extracorporeal Cell and Gene Therapy Delivery Technologies to the Biopharma industry
Cell and Gene Therapy is changing rapidly and becoming increasingly competitive as Biopharma companies pursue similar targets and diseases.
Lupagen’s extracorporeal delivery experts can collaborate with you to create and expand the value of your core cell and gene therapy program with a differentiated and accelerated path to the clinic.
CASE STUDIES
In vivo CGT Company expanding indication reach through Extracorporeal delivery.
Customer Challenge
Systemic Administration of lentiviral vectors required high vector dose creating safety risks
Biotech company with an in situ lentiviral gene delivery vehicle needed a new route of administration that could control dose and limit unbound particles in vivo.
Lupagen Solution
Alternative route of administration that mitigates dosing challenges
- Designed rapid feasibility testing to optimize lentiviral vector for use in Lupagen extracorporeal system.
- Prepared for key regulatory agency meetings that provided clear guidance and path forward for bedside delivery of viral vector to generate CAR-T cells in vivo.
Biotech company now has a viable preclinical alternative to control dose and mitigate safety risks relative to other in vivo CAR-T cell programs
Key Services
- Extracorporeal process optimization for lentiviral vectors
- PoC Studies
- Regulatory Agency Meetings
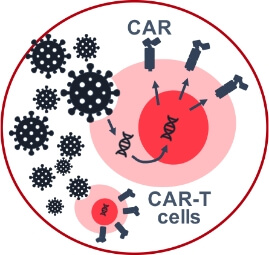
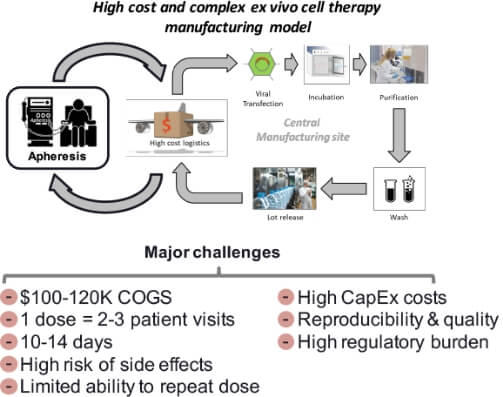
Biotech company with an Ex-Vivo Autologous Cell Therapy technology exploring Extracorporeal Delivery to expand patient access into new indications and markets
Customer Challenge
Biotech company’s central manufacturing process is too costly and complex limiting potential to address broader autoimmune indications
Biotech company with a first-in-class platform technology enabling the treatment of Autoimmune indications had developed a successful ex-vivo approach. However, the cost structure limits commercial viability in larger indications.
Lupagen Solution
Lupagen’s bedside extracorporeal approach presented an ideal alternative to bring cost of goods down to biologics pricing and ability to access larger patient populations and markets
Lupagen collaborated with customer to select and screen viral vector candidates that are optimized for extracorporeal delivery and develop studies to enable path to clinic.
Lupagen has provided Biotech company with a potential opportunity to expand into broader autoimmune indications driven by lower costs of goods and simpler production process compared to other CGT technologies
Key Services
- In situ Lentiviral Vector Screening
- Extracorporeal process optimization for lentiviral vectors
- PoC Studies
Biotech Company expanding therapeutic opportunities to apply their mRNA platform technology into new indications and markets
Customer Challenge
Biotech company with novel mRNA technology is facing challenges with targeted delivery and repeat dosing for mRNA-based gene therapy.
mRNA therapeutics have the advantage of transient expression appropriate for chronic conditions but require ability to repeat dose. Biotech company needed a new route of administration that can significantly lower dose, mitigate biodistribution challenges and lower immunogenicity to enable repeat dosing.
Lupagen Solution
Alternative route of administration that mitigates dosing challenges
Lupagen collaborated with customer to select and screen lipid nanoparticle candidates that are optimized for in situ gene delivery and develop studies to help enable path to clinic.
Lupagen has provided Biotech company with a new potential regulatory and clinical pathway for an mRNA-based therapeutic by enabling controlled and repeat dosing
Key Services
- Lipid nanoparticle Screening
- Extracorporeal process optimization for LNPs
- PoC Studies
CUSTOMERS
Making A Difference: Who We Serve
Lupagen’s proprietary delivery technology is available to select biopharma companies developing CGT requiring targeted peripheral blood mononuclear cells for preclinical development
- Selected Customer programs (non-exhaustive)
- Therapeutic area
- Gene delivery technology
- CAR T-cell
- Oncology
- Lentiviral
- Confidential
- Autoimmune
- Lentiviral
- CAR T-Cell
- Autoimmune
- Lentiviral
- CAR T-Cell
- Oncology
- LNP-mRNA
- TCR
- Oncology
- Lentiviral
- Gene Editing
- Rare Disease
- LNP-CAS9
News and Insights